Immunohistochemical Staining Immunohistochemistry (IHC) is a technique for identifying cellular or tissue constituents (antigens) by means of antigen-antibody interactions, the site of antibody binding being identified either by direct labelling of the antibody, or by use of a secondary labelling method.
In Situ Hybridization (ISH) techniques allow the demonstration of specific nucleic acid sequences (genes) in their cellular environment.
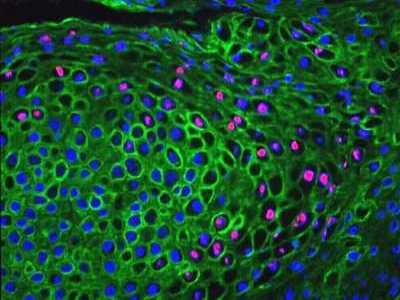
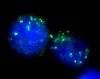
Above: Human Papillomavirus DNA demonstrated by In Situ Hybridisation (pink) in epithelial cells identified by indirect immunofluorescence using antibody against cytokeratin (green).
Immunohistochemistry (IHC) combines anatomical, immunological and biochemical techniques for the identification of specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes it possible to visualize the distribution and localization of specific cellular components within a cell or tissue. The term immunohistochemistry is often used interchangeably with immunocytochemistry and immunostaining.
Coons and his colleagues bound a fluorescent marker to an antibody and used the complex to identify antigens in tissue sections. Despite their relative age, the fluorescent probes are still widespread in use and with the advent of confocal microscopy, are experiencing a renaissance. The basic immunocytochemical philosophy described by Coons and his colleagues of an antibody linked to a microscopically dense marker, has not altered, but the microscopically dense marking system has been developed for applications to a wide range of histological and electron microscope techniques, improvements have been made in protein conjugation, tissue fixation methods, detection labels and microscopes, making immunohistochemistry a routine and essential tool in many laboratories.
Nakane and Pierce, and Avrameas and Uriel, covalently linked the enzyme peroxidase, with the second antibody for use in histology. The peroxidase can be visualized by development with one of several different substrates to produce a brown, blue, or yellow reaction product. An increased sensitivity of the immunoperoxidase technique was achieved with the unlabelled antibody peroxidase anti-peroxidase (PAP) technique. The marker is a peroxidase complex of three proxidase molecules associated with two anti-peroxidase molecules. This technique gives a high signal, since three peroxidase molecules are associated with each antigen. Alkaline phosphatase can be used in a similar manner, alkaline phosphatase anti-alkaline phosphatase (APAAP) technique.
Many of the immunochemical staining methods in use are based on the high affinity that (strept)avidin (Streptomyces avidinii) and avidin (chicken egg) have for biotin. Both possess four binding sites for biotin, but due to the molecular orientation of the binding sites, fewer than four molecules of biotin will actually bind. Because avidin is a glycoprotein and has an isoelectric point (pI) of 10, it has a propensity to non-specifically bind to lectin-like and negatively charged tissue components at physiological pH. It has been largely replaced today by streptavidin.
The inherent amplification of sensitivity made the avidin and streptavidin-biotin methods more desirable than the previously described PAP and APAAP methods.The basic sequence of reagent application consists of primary antibody, biotinylated secondary antibody, followed either by the preformed (strept)avidin-biotin-enzyme complex of the avidin-biotin complex (ABC) technique or by the enzyme-labelled streptavidin. Both conclude with the substrate solution. Horseradish peroxidase and alkaline phosphatase are the most commonly used enzyme labels. While the authors of the ABC method reported this procedure to have a greater sensitivity than the PAP method, Giorno subsequently found the sensitivity of a labelled avidin-biotin (LAB)method to be approximately four- to eight-fold greater than the ABC method. In both methods, the avidin has now been largely replaced by the use of streptavidin leading to the labelled streptavidin-biotin (LSAB) method and a modified ABC procedure, respectively.
One of the important goals in immunohistochemistry is to achieve greater sensitivity with detection systems using the shortest possible incubation time. However, multi-step detection systems have several drawbacks, such as complex time-consuming protocols, difficulties in standardization, suboptimal detection of hard to detect antigens, endogenous biotin activity, etc. Recently, new detection systems have been introduced using natural or synthetic polymer carriers that are coupled to linker antibodies. This approach increases the number of available enzymes or ligands binding at the antigenic site, thus increasing their reactivity with the chromogen. Because these systems avoid the use of (strept)avidin and biotin, nonspecific staining as a result of endogenous biotin is eliminated.
