OptiView DAB IHC Detection Kit
Ventana's OptiView DAB IHC Detection Kit (OptiView) is an indirect, biotin-free system for detecting mouse IgG, mouse IgM and rabbit primary antibodies. OptiView uses a proprietary, non-endogenous hapten system to provide enhanced sensitivity and specificity.
OptiView's exclusive software gives users more control over their IHC protocol than ever before on the BenchMark family of instrumentation. This control allows OptiView to be optimized for any need ranging from very low-expressing antigens to shorter turnaround times.
Ventana Medical Systems, Inc. launches first fully automated IHC detection kit with customizable detection chemistry.
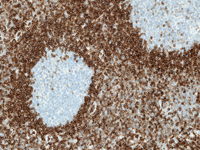
Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group, announced today the launch of their OptiView DAB IHC Detection Kit, the first fully-automated immunohistochemistry (IHC) detection kit that combines both sensitive chemistry and flexible software to deliver highly customizable staining. OptiView Detection significantly enhances stain quality, allowing pathologists to view IHC assays more clearly than ever before.
“OptiView detection is an important, innovative solution to help pathologists be confident that even with the most difficult-to-view markers, they are making the right diagnoses and driving the right patient treatments,” says Greg Yap, Head of Advanced Staining Assays for Ventana. “This high-performance detection system is a perfect complement to current and future VENTANA assay solutions that enable pathologists to contribute to the best patient outcomes in the era of personalized healthcare.”
The combination of enhanced chemistry and flexible software of the OptiView detection system leads to quicker turnaround time for slides and allows more flexibility for labs to customize assays to their visible preferences and performance characteristics. OptiView detection meets the ever-changing technological needs of pathologists, providing increased medical value and efficiency gains through improved laboratory workflow, enabling labs to report patient results sooner.
Visit the Ventana website to view a sample OptiView image.
“The novel, proprietary OptiView chemistry features dramatically enhanced sensitivity without increased background. This helps pathologists visually identify even very low levels of protein expression in tumor samples without interference from non-specific staining,” says Eric Walk, Chief Medical Officer for Ventana. “This gives pathologists stronger confidence in their diagnoses, driving better decisions for patient care.”
The OptiView kit is compatible with the entire line of VENTANA BenchMark instruments - integrating completely into the Ventana goal of better solutions for patient care and continuous medical innovation.
About Ventana Medical Systems, Inc.
Ventana Medical Systems, Inc. (“VMSI”) (SIX: RO, ROG; OTCQX: RHHBY), a member of the Roche Group, innovates and manufactures instruments and reagents that automate tissue processing and slide staining for cancer diagnostics. VENTANA solutions are used in clinical histology and drug development research laboratories worldwide. The company’s “Smart Systems” - intuitive, integrated staining and workflow management platforms that optimize laboratory efficiencies to reduce errors - support diagnosis and inform treatment decisions for anatomic pathology professionals. Together with Roche, VMSI is driving personalized medicine through accelerated drug discovery and the development of “companion diagnostics” to identify the patients most likely to respond favorably to specific therapies. Visit www.ventana.com to learn more.
